Dr. Stephen Perusich, Senior Scientist at Cella Energy’s Kennedy Space Center, Florida location recently sat down with Space Safety Magazine to talk about his company’s creative new method for storing hydrogen fuel in reusable plastic pellets. Dr. Perusich manages the lab at the Space Life Sciences Laboratory facility, where new hydrogen storage methods are in the research and development phase. Making hydrogen readily available as a clean, alternative fuel and storing it in a form at room temperature may be the solution to safer, greener transportation. Hydrogen has the highest energy density per unit mass compared to fossil fuels.
That solution comes in the form of a pellet you can hold in the palm of your hand. This method, invented by Cella Energy co-founder Professor Stephen Bennington and his students in England, stores the hydrogen from ammonia borane and other hydrides inside linear chains of a polyethylene oxide polymer. Similar to the structure of a rope, the linear chains hold the hydrogen compound in place. By freeze drying the two together, the ammonia borane and polyethylene oxide can be made into a fine powder that is then shaped into cylindrical pellets. Coating the pellets with a layer of plastic for filtering makes them safe for handling. It is a clean, efficient way to store the hydrogen without use of a coolant or insulation. The method can also be used to create light, thin fibers that resemble cotton balls that you would buy at the pharmacy.
The Safety Of Hydrogen
At the beginning of the interview, Dr. Perusich produced two small glass vials and opened their contents onto the table in front of him. In one vial were approximately 10 small white beads and in the other, a small square of fluffy fibers.
Dr. Perusich: Here, you can handle them very easily if you want to.
Space Safety Magazine (SSM): Are they toxic to the skin?
Dr. Perusich: No, not at all. They have a plastic coating over them. If you took a dozen or so of these, you could fill up your gas tank, if it was a hydrogen tank, and run your car for 300 miles or so.
SSM: The cotton swab in this vial, is it also an ammonia borane compound?
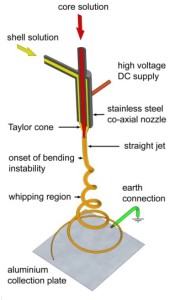
Cella’s electrospinning process uses a strong electric field (E) to spin and draw composite fibers from the polymer and hydride solutions. (Credits: S. Perusich).
Dr. Perusich: Yes, it’s made by the original electrospun process by which the fibers are created. The hydrogen goes in the empty space inside the fibers. This is the original method that Steve Bennington in England came up with about 5 years ago with some of his students. It essentially puts a core hydride material in the middle and a polymer shell solution on the outside. A high voltage is placed between the nozzle and a grounding plate to draw, or stretch the fibers and decrease their diameters. The electrospun fibers are far smaller in diameter than a human hair. If you look at it on a cross-section of the fibers, you see a bunch of holes inside so it’s not exactly core sheath, but instead a composite. From these fibers, we can make this stuff here (points to the fiber square) or by variations on that process, we can make this stuff here (holds up a pellet) and that’s essentially what we make. What looks like cotton is actually very dense amount of fibers spun from the chemicals that store the hydrogen.
SSM: Where does the vision of hydrogen fuel research stem from?
Dr. Perusich: The concept for a hydrogen economy goes back 100 years or so. The question has been: how can we make, store, and use hydrogen in a practical manner to maximize its energy potential in society? Up to today, we’ve only had two ways of storing hydrogen. One is as compressed gas, as some cars in California use. The other way is to cool it down to a liquid, which is what we do here at the Kennedy Space Center. Liquid hydrogen is great for rockets but isn’t really practical for cars. Even at KSC, by the time we obtain the hydrogen gas, cool it down and then use it in a rocket launch, 50% of it will be lost to the atmosphere. Eventually, I’d love to use Cella Energy’s technology to actually power rockets. Instead of continuing to keep the hydrogen cryogenic- in a liquid state and suffering tremendous losses of hydrogen during launch delays, we could instead place a bin of our pellets next to the rocket at room temperature. Then, moments before launch, we could heat the vessel, release the hydrogen into the rocket, and away she goes. However, that’s in the future.
Hydrogen fuel research came about in a serious manner in the 1900’s, around the time the first cars were being developed. There were three kinds of cars back then- electric, steam-powered and the oil or gasoline powered car. Steam had lots of problems and that was eliminated early on. Electric cars didn’t have efficient batteries or fuel cells at the time, so they lost out to gasoline. There has been a debate since that time: did we go down the wrong path?
It is difficult to beat the energy density of gasoline on a per volume basis. Hydrogen will never compete with gasoline based on this. It will, however, compete with battery technology, like lithium batteries, and can be two or three times better than battery technology. The beautiful part about hydrogen is that it’s clean. When you put it into a fuel cell and react it, you get water out. So that’s a huge environmental advantage.
SSM: What function does the lab here at the Kennedy Space Center have in the hydrogen storage research project?
Dr. Perusich: We have a lab of 15 people in Oxford, England and a lab of 4 researchers here in the United States, where 90% of the personnel have their Ph.D. We tend to joke that anything that’s on the ground they do and anything that’s in the sky, we do. That’s a little simplistic because a lot of the research that each lab does helps the other lab. However, any kind of airplane/space related work, including unmanned aerial vehicles (UAV’s) and radiation shielding, we do here. The lab in England right now is working on material development to optimize the properties of these pellets and fibers. In the USA, we are looking at a way to regenerate these pellets. Doing so will drive down the price. We try to look at the supply chain all the way back to the raw ingredients like boron and hydrogen and several boron complexes. Right now the pellets are not reusable, but we hope to remedy that soon.
[cleveryoutube video=”OXAf7MsuPjI” vidstyle=”1″ pic=”” afterpic=”” width=”” quality=”inherit” starttime=”” endtime=”” caption=”” showexpander=”off” alignment=”left” newser=””]
Storing Energy in Plastic Pellets
SSM: How many years in development was the final product- hydrogen stored in polyethylene oxide and plastic pellets?
Dr. Perusich: It goes back 5 years to some laboratory work, at Oxford University, Imperial College London and Rutherford Appleton Laboratory. They were looking for a way to penetrate the American market and Space Florida approached them since KSC is the single largest user of hydrogen in the world. We and Space Florida saw the potential of using our technology for both radiation shielding as well as rocket propulsion. That’s how we really got started here. We hope to have a commercial shielding product available in just 2-3 years.
SSM: How long does it take to process one pellet or a batch of these pellets?
Dr. Perusich: We are researching three processes right now. All three work but are not yet optimized. Right now, all of this is done by hand. To make one pellet takes roughly a half an hour, maybe less. To spin this amount of fiber takes one hour. For pellets in a car engine, we would make them 1000 times smaller than our current pellet in order to act more as a fluid than a solid. One thousand smaller pellets could to fit on your finger nail. We could then pump these small pellets as we do gasoline now.
SSM: Is the hydrogen pellet creation process dangerous? What steps does your lab take to ensure worker safety?
Dr. Perusich: That’s a great question. If you had the raw hydride in air at normal room conditions, it has the potential to catch fire. Therefore, we do all our chemical processing in the dry glove box and we don’t move anything outside of it until we’re done processing the completed composite and we are sure it’s safe. To establish a manufacturing process, we will be using a lab similar to an electronics clean room.

Cella Energy’s mock-up demonstrating a pure hydrogen fuel station for automobiles with dual nozzle pumps. (Credits: S. Perusich).
SSM: So, a handful of these smaller ones would power the car?
Dr. Perusich: Yes, absolutely. What we envision is a customer pulling up to a “gas station” but not a gasoline station. It will have a nozzle with two ports in it, one to suction out the used pellets and one to put in new pellets. In current gasoline stations, tankers bring gasoline and then go back empty. Here, they go back full of used pellets ready to reprocess them. We’ve been talking with the biggest car companies here in America and in Germany and there is a lot interest. We also have some programs with airplane companies for the same applications.
SSM: Cella Energy has both UK-based and a US-based facilities. Based on your company’s environmental research, which country has a larger need for alternative cleaner fuels?
Dr. Perusich: I can’t tell you which one needs it more, but the product will roll out in Europe first. The first reason is because of more extensive environmental legislation in Europe. The second is fuel price; gasoline in England is above $8.00/gallon. In the US, we do not have the same sense of environmental urgency as other countries because of our vast natural resources.
SSM: At what temperature is the hydrogen is released?
Dr. Perusich: About 100 degrees Celsius At that temperature, the pellet stays intact. At a higher temperature, you can get more hydrogen out but eventually it will melt to goo. Up to 100 or 120 degrees C, it stays intact and you should be able to recycle it. We want to use waste energy there to do the heating inside the engine block to provide a heating source. The whole thought here is to take our material, put it in a tank and when needed, take the right amount of hydrogen pellets into a pre-heater to get pure hydrogen out. Then we flow the released hydrogen gas into either an internal combustion engine or fuel cell, react it, and take the spent material out. That should give you nice, clean hydrogen energy and spent pellets we can regenerate.
In addition, for diesel engines it’s been known for a while that if you a few percent hydrogen to a diesel engine, the efficiency goes up. One way to crack into the market is to start with that technology. Better efficiency and lower emissions- hey!
SSM: Liquid hydrogen has previously been used in the Space Shuttle main engine. How does the energy density of the plastic pellets compare to that of liquid hydrogen?
Dr. Perusich: Since we are diluting our hydrogen in plastic, it has a lower energy density. The hydrogen quality that would come off would be comparable to what we use right now but would need more to create the same amount. The advantage of the pellets is that they keep for an infinite amount of time next rocket on the pad without getting those losses seen in the liquid form. In addition, only the hydrogen gas would enter and be used by the rockets, so no additional weight would be added during launch. The pellets would stay behind.
Use If Hydrogen Pellets for Radiation Shielding
SSM: Hydrogen nano-compounds can also be used for shielding electronics from the hazards of space. The commercial satellite market is an obvious choice to advertise this technology to. How does Cella’s shielding method compare to shielding with liquid hydrogen?
Dr. Perusich: Hydrogen gives you an excellent shielding medium. Polyethylene isn’t bad. Aluminums and metals are not very good shields for the most part because protons come in and fracture the metal atoms, causing highly energetic particles. This secondary radiation is worse than the primary radiation. Our materials are in between pure hydrogen and polyethylene. Dealing with liquid hydrogen, much energy must be put into cooling, and the problem of hydrogen escape is always present. Ultrahigh molecular polyethylene is used for shielding on the International Space Station. It’s about 6 ½ cm thick in the crew quarters. Most radiation is stopped in this area except the really high energy particles above 100 eV. Our material has 30% more hydrogen than polyethylene does and gives you a 40% lower radiation dose than polyethylene does.
Now that Cella has a viable product, they will be continuing to research how plastics and hydrogen might shield astronauts from space radiation, a subject that is vital to enabling deep space exploration and has yet to produce a feasible solution. With initial data from NASA, Cella researchers are investigating the effectiveness of the shielding against radiative protons similar to those encountered in low Earth orbit. In the next 2-3 years, Cella will be taking its hydrogen pellets to the commercial markets, where real-world applications will develop the technology even further.





























































![A trajectory analysis that used a computational fluid dynamics approach to determine the likely position and velocity histories of the foam (Credits: NASA Ref [1] p61).](http://www.spacesafetymagazine.com/wp-content/uploads/2014/05/fluid-dynamics-trajectory-analysis-50x50.jpg)



I think radiation protection is a must, that’s why it’s great to see the pellets incorporating radiation protection!
Right you are….radiation is one of the biggest barriers to deep space flights and still looking for clever solutions.